Seznamy Atom Economy Examples
Seznamy Atom Economy Examples. Consider the combustion of … Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.
Prezentováno Chemistry Atom Economy And Percentage Yield
Please do not block ads on this website. Q the examples above clearly demonstrate the environmental (and economical) Worked example hydrogen can be manufactured by reacting methane with steam: Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m.Hydrogen can be manufactured by reacting methane with steam:
This is illustrated by using the blast furnace reaction from example 14.2a.3 above. This is illustrated by using the blast furnace reaction from example 14.2a.3 above. An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. How to calculate atom economy step 1. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Calculate the relative molecular mass of each of the products. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:

Calculating the percene atom economy of a reaction. Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: Percentage yield is calculated from the mass of reactants and the mass of products. No ads = no money for us = no free stuff for you! Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Write out the balanced equation. How to calculate atom economy step 1. 14.01.2019 · which is an example of the atom economy?

Calculate the relative molecular mass of each of the products. As in the previous examples one can set up an atom economy table and calculate the % atom economy. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Green chemists define atom economy as: Using the atomic masses of fe = 56, … An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Usually the atom economy is less than 100%. Calculate the relative molecular mass of each of the products.

No ads = no money for us = no free stuff for you! Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Hydrogen can be manufactured by reacting methane with steam: No ads = no money for us = no free stuff for you! Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. The greater the value of the %atom economy, the less the amount of waste product produced. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Green chemists define atom economy as: Let's do some examples of simple reactions. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. This is illustrated by using the blast furnace reaction from example 14.2a.3 above. Calculating the percene atom economy of a reaction.

Q the examples above clearly demonstrate the environmental (and economical) Q the examples above clearly demonstrate the environmental (and economical)

Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Calculate the relative molecular mass of each of the products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Green chemists define atom economy as: Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. As in the previous examples one can set up an atom economy table and calculate the % atom economy. The greater the value of the %atom economy, the less the amount of waste product produced. How to calculate atom economy step 1... How to calculate the atom economy of iron?

Write out the balanced equation. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. The greater the value of the %atom economy, the less the amount of waste product produced. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. 14.01.2019 · which is an example of the atom economy? Usually the atom economy is less than 100%. Worked example hydrogen can be manufactured by reacting methane with steam:. Q the examples above clearly demonstrate the environmental (and economical)

Usually the atom economy is less than 100%... Usually the atom economy is less than 100%. Consider the combustion of … Calculate the relative molecular mass of each of the products. Hydrogen can be manufactured by reacting methane with steam: Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. This is illustrated by using the blast furnace reaction from example 14.2a.3 above. How to calculate the atom economy of iron? Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction.. 14.01.2019 · which is an example of the atom economy?

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. 14.01.2019 · which is an example of the atom economy? Green chemists define atom economy as: Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Hydrogen can be manufactured by reacting methane with steam: Calculating the percene atom economy of a reaction.. Atom economy for the process is low, at 51%.

Usually the atom economy is less than 100%. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. No ads = no money for us = no free stuff for you! Green chemists define atom economy as: Write out the balanced equation. Hydrogen can be manufactured by reacting methane with steam: The greater the value of the %atom economy, the less the amount of waste product produced... Worked example hydrogen can be manufactured by reacting methane with steam:

Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Consider the combustion of … The greater the value of the %atom economy, the less the amount of waste product produced. Q the examples above clearly demonstrate the environmental (and economical) 14.01.2019 · which is an example of the atom economy? Please do not block ads on this website.

As in the previous examples one can set up an atom economy table and calculate the % atom economy. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Please do not block ads on this website. 14.01.2019 · which is an example of the atom economy?. Worked example hydrogen can be manufactured by reacting methane with steam:

An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. Green chemists define atom economy as: Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. No ads = no money for us = no free stuff for you! As in the previous examples one can set up an atom economy table and calculate the % atom economy. Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Calculate the relative molecular mass of each of the products... The greater the value of the %atom economy, the less the amount of waste product produced.

Using the atomic masses of fe = 56, …. Consider the combustion of … Let's do some examples of simple reactions. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. How to calculate atom economy step 1. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. How to calculate atom economy step 1.

Calculating the percene atom economy of a reaction.. Usually the atom economy is less than 100%. Percentage yield is calculated from the mass of reactants and the mass of products. No ads = no money for us = no free stuff for you! Worked example hydrogen can be manufactured by reacting methane with steam: Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Consider the combustion of … Write out the balanced equation. Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. How to calculate the atom economy of iron?

How to calculate the atom economy of iron? No ads = no money for us = no free stuff for you! Green chemists define atom economy as: Worked example hydrogen can be manufactured by reacting methane with steam: Percentage yield is calculated from the mass of reactants and the mass of products. Q the examples above clearly demonstrate the environmental (and economical)

Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. The greater the value of the %atom economy, the less the amount of waste product produced. Calculate the relative molecular mass of each of the products. Please do not block ads on this website. Let's do some examples of simple reactions. Calculating the percene atom economy of a reaction. Let's do some examples of simple reactions.

Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... This is illustrated by using the blast furnace reaction from example 14.2a.3 above... Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction.
How to calculate atom economy step 1.. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Please do not block ads on this website. Percentage yield is calculated from the mass of reactants and the mass of products. How to calculate the atom economy of iron? Write out the balanced equation. Green chemists define atom economy as:

How to calculate atom economy step 1... Worked example hydrogen can be manufactured by reacting methane with steam:
Worked example hydrogen can be manufactured by reacting methane with steam: Worked example hydrogen can be manufactured by reacting methane with steam: Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Calculate the relative molecular mass of each of the products. Percentage yield is calculated from the mass of reactants and the mass of products. Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m.
Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. . Q the examples above clearly demonstrate the environmental (and economical)
Worked example hydrogen can be manufactured by reacting methane with steam: In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: How to calculate the atom economy of iron? Usually the atom economy is less than 100%.. This is illustrated by using the blast furnace reaction from example 14.2a.3 above.

Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. The greater the value of the %atom economy, the less the amount of waste product produced. Consider the combustion of …. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy.
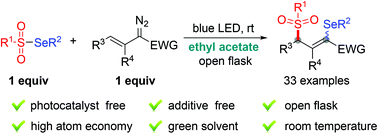
How to calculate atom economy step 1. An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. How to calculate the atom economy of iron? Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Green chemists define atom economy as: Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Using the atomic masses of fe = 56, … Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. As in the previous examples one can set up an atom economy table and calculate the % atom economy.

Calculating the percene atom economy of a reaction.. Write out the balanced equation. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Let's do some examples of simple reactions. Calculate the relative molecular mass of each of the products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. The greater the value of the %atom economy, the less the amount of waste product produced. Please do not block ads on this website.

Calculate the relative molecular mass of each of the products. Worked example hydrogen can be manufactured by reacting methane with steam: Q the examples above clearly demonstrate the environmental (and economical) An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. Hydrogen can be manufactured by reacting methane with steam: Consider the combustion of … Please do not block ads on this website. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy.

Usually the atom economy is less than 100%. Hydrogen can be manufactured by reacting methane with steam: Consider the combustion of … Calculating the percene atom economy of a reaction. How to calculate the atom economy of iron? Q the examples above clearly demonstrate the environmental (and economical) Usually the atom economy is less than 100%. Calculate the relative molecular mass of each of the products. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Using the atomic masses of fe = 56, … Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Please do not block ads on this website. Usually the atom economy is less than 100%. Percentage yield is calculated from the mass of reactants and the mass of products. Q the examples above clearly demonstrate the environmental (and economical) The greater the value of the %atom economy, the less the amount of waste product produced... No ads = no money for us = no free stuff for you!

Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: The greater the value of the %atom economy, the less the amount of waste product produced. Please do not block ads on this website. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. No ads = no money for us = no free stuff for you! Using the atomic masses of fe = 56, …. Percentage yield is calculated from the mass of reactants and the mass of products.

Calculate the relative molecular mass of each of the products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Hydrogen can be manufactured by reacting methane with steam: Please do not block ads on this website. This is illustrated by using the blast furnace reaction from example 14.2a.3 above. Consider the combustion of … Write out the balanced equation. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Using the atomic masses of fe = 56, … Usually the atom economy is less than 100%. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy... Worked example hydrogen can be manufactured by reacting methane with steam:

14.01.2019 · which is an example of the atom economy? Let's do some examples of simple reactions.

Hydrogen can be manufactured by reacting methane with steam: This is illustrated by using the blast furnace reaction from example 14.2a.3 above. Green chemists define atom economy as: Q the examples above clearly demonstrate the environmental (and economical) Worked example hydrogen can be manufactured by reacting methane with steam: 14.01.2019 · which is an example of the atom economy?

How to calculate atom economy step 1.. Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. How to calculate atom economy step 1. Let's do some examples of simple reactions. Let's do some examples of simple reactions.

Atom economy for the process is low, at 51%... Using the atomic masses of fe = 56, … Q the examples above clearly demonstrate the environmental (and economical).. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Consider the combustion of … Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1.. Green chemists define atom economy as:

An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Green chemists define atom economy as: Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Consider the combustion of … Worked example hydrogen can be manufactured by reacting methane with steam: Let's do some examples of simple reactions. An alternative synthesis of nitrobenzene, employing a triflate catalyst, has recently been established on a laboratory scale. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244.

Usually the atom economy is less than 100%... Worked example hydrogen can be manufactured by reacting methane with steam:. The greater the value of the %atom economy, the less the amount of waste product produced.

Atom economy for the process is low, at 51%. Please do not block ads on this website. Consider the combustion of … How to calculate the atom economy of iron? How to calculate atom economy step 1. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Q the examples above clearly demonstrate the environmental (and economical) Write out the balanced equation. The greater the value of the %atom economy, the less the amount of waste product produced. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Using the atomic masses of fe = 56, …. Usually the atom economy is less than 100%.

14.01.2019 · which is an example of the atom economy?.. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Worked example hydrogen can be manufactured by reacting methane with steam: As in the previous examples one can set up an atom economy table and calculate the % atom economy. Please do not block ads on this website. Using the atomic masses of fe = 56, … Out of a total mass of reactants or products of 160 + 84 = 112 + 132 = 244. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Green chemists define atom economy as:.. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:

If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products... Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m. Let's do some examples of simple reactions. In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Consider the combustion of … Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Green chemists define atom economy as: If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Calculating the percene atom economy of a reaction.
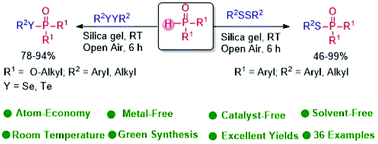
Atom economy and reaction m anium catalyzed cyano borrowing calculations in chemistry 2 124kb harnessing the catalytic behaviour of 1 atom economy and reaction m... Let's do some examples of simple reactions. Using the atomic masses of fe = 56, … Calculate the relative molecular mass of each of the products. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. How to calculate atom economy step 1.

This is illustrated by using the blast furnace reaction from example 14.2a.3 above. .. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Percentage yield is calculated from the mass of reactants and the mass of products. As in the previous examples one can set up an atom economy table and calculate the % atom economy. Atom economy for the process is low, at 51%. How to calculate atom economy step 1. Calculating the percene atom economy of a reaction.
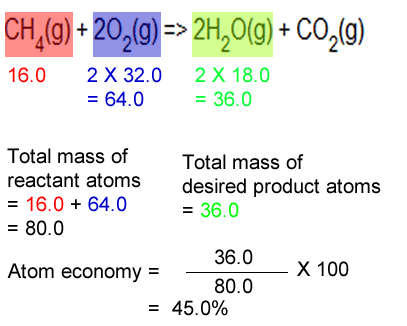
Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction... Worked example hydrogen can be manufactured by reacting methane with steam: Hydrogen can be manufactured by reacting methane with steam: Using the atomic masses of fe = 56, … In addition reactions, the atom economy will always be 100%, because all of the atoms are used to make the desired product whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Green chemists define atom economy as: Although the acid (h +) used in this reaction is not incorporated into the desired product it is used only in catalytic amounts and therefore indicated in black (not green or brown) and it is not considered in the atom economy table or the calculation of the atom economy. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.. The greater the value of the %atom economy, the less the amount of waste product produced.

Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Atom economy for the process is low, at 51%.. The greater the value of the %atom economy, the less the amount of waste product produced.

Percentage yield is calculated from the mass of reactants and the mass of products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. How to calculate atom economy step 1. The greater the value of the %atom economy, the less the amount of waste product produced. Usually the atom economy is less than 100%. Consider the combustion of … No ads = no money for us = no free stuff for you! Let's do some examples of simple reactions. Q the examples above clearly demonstrate the environmental (and economical) Write out the balanced equation.. As in the previous examples one can set up an atom economy table and calculate the % atom economy.

Worked example hydrogen can be manufactured by reacting methane with steam: .. As in the previous examples one can set up an atom economy table and calculate the % atom economy.

How to calculate the atom economy of iron?. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. The greater the value of the %atom economy, the less the amount of waste product produced. How to calculate atom economy step 1. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Calculate the relative molecular mass of each of the products. Hydrogen can be manufactured by reacting methane with steam: Usually the atom economy is less than 100%. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.. Let's do some examples of simple reactions.

Worked example hydrogen can be manufactured by reacting methane with steam: Worked example hydrogen can be manufactured by reacting methane with steam: Q the examples above clearly demonstrate the environmental (and economical) The greater the value of the %atom economy, the less the amount of waste product produced. Calculating the percene atom economy of a reaction... Green chemists define atom economy as:

Q the examples above clearly demonstrate the environmental (and economical) This is illustrated by using the blast furnace reaction from example 14.2a.3 above.
